RENOVA SPINE™ 8G
Kyphoplasty kit
- Minimally invasive surgery: patient recovers faster;
- Percutaneous: less invasive and less traumatic treatment;
- Cavity creation: minimized risk of cement leakage;
- Vertebral body height restoration;
- No metallic implants left in situ;
- Reduction in morbidity and fast functional recovery;
- Suitable from the lower thoracic to the lumbar vertebrae.
A vertebral compression fracture (VCF) occurs when the vertebral body fractures and collapses due to osteoporotic conditions, tumours (e.g. myeloma) or trauma.
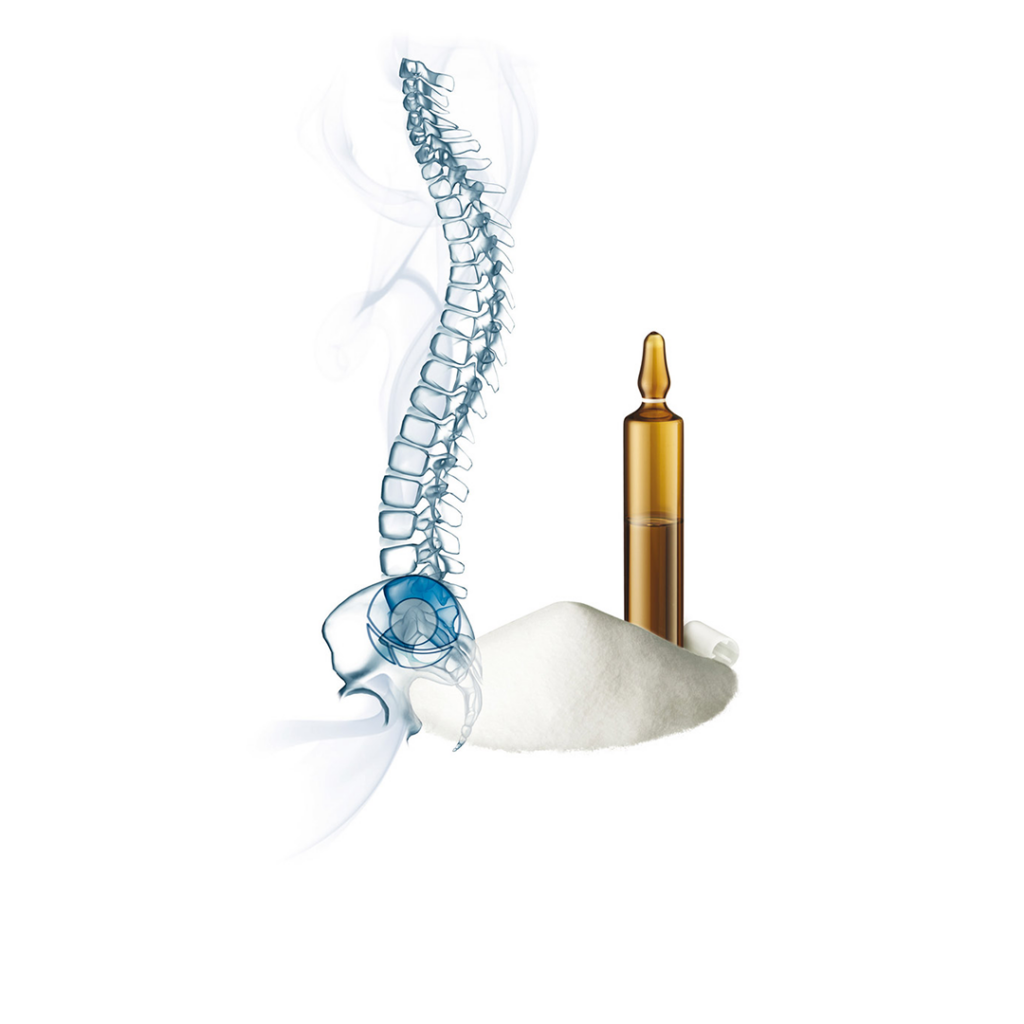
The principle of kyphoplasty is to restore vertebral body anatomy gently and progressively by inflating balloons and then reinforcing the vertebra with cement. The balloons create a cavity within the vertebral body, compressing the cancellous bone and thus limiting the risk of cement leakage from the vertebral body.
The balloon catheter is then deflated, and the cavity created is now filled with bone cement, avoiding any leakage.
RENOVA SPINE™ 8G system allows performing a Kyphoplasty procedure with a classic approach.
Surgical procedure – CLASSIC APPROACH
Approach & Initial Access
- The approach can be extrapedicular or transpedicular.
- Insert the bone access needle, then remove its stylet.
- Under fluoroscopy, insert the Kirschner wire through the needle’s cannula, positioning its tip ~5mm from the anterior vertebral wall (lateral view).
- Remove the bone access needle, leaving the guide wire in place.
- Insert the funnel-shaped working cannula & expander, allowing the guide wire to slide inside.
- Hold the working cannula in place and carefully remove the guide wire.
- Extract the inner funnel-shaped cannula, leaving only the outer working cannula inside the patient.
Creating the Pathway
- Insert the drill through the working cannula and into the vertebral body.
- Rotate 180° clockwise and counterclockwise to create a pathway for the balloon, then remove the drill.
Balloon Inflation
- Connect the balloon catheter to a preloaded inflation system (contrast medium-to-saline ratio: 1:2).
- Under fluoroscopic guidance, insert the balloon catheter through the working cannula and position it correctly (AP & lateral view).
- Inflate the balloon to the desired volume or maximum measurable pressure, then fully deflate by unscrewing the handle of the inflation device.
- Remove the deflated balloon catheter.
Cement Preparation & Injection
- While inflating the balloon, begin cement preparation, if necessary.
- Load Luer-lock syringes with bone cement and transfer it into a bone filler.
- Inject cement through the working cannula, using a plunger for controlled delivery.
- Once the cement is placed, remove the bone filler, reinsert the stylet, and carefully extract the working cannula.
| TOOLS | SINGLE COMPONENTS CODES | KIT FOR MONOLATERAL APPROACH | KIT FOR BILATERAL APPROACH |
| RENOVA SPINE™ kyphoplasty bone access needle | RESOB1110T | 1 | 2 |
| RENOVA SPINE™ Kyphoplasty working cannula and expander | RESWT0812C | 1 | 2 |
| RENOVA SPINE™ kyphoplasty Kirschner guide trocar tip | RESFG1128T | 1 | 2 |
| RENOVA SPINE™ kyphoplasty Kirschner guide flat tip | RESFG1128P | 1 | 2 |
| RENOVA SPINE™ kyphoplasty drill | RESDRI0812C | 1 | 1 |
| RENOVA SPINE™ balloon catheter | 10 mm: RESB10PM – RESB10J | 1 | 2 |
| 15 mm: RESB15PM – RESB15J | |||
| 20 mm: RESB20PM – RESB20J | |||
| RENOVA SPINE™ bone filler | RESFIL0812C1 | 3 | 6 |
| Syringes (2,5 ml) | SI02.5L/L-A | 4 | 4 |
| Inflation device: digital or analog | Digital: K05-03069 | 1 | 2 |
| Analog: INFLA30 |