BONE CEMENT
Bone cement designed for spinal applications
SPINOS fulfills all requirements for bone cement in spinal surgery.
- Versatile use – Suitable for vertebroplasty, kyphoplasty and pedicle screws;
- Fast preparation;
- Reduced leakage risk;
- Extended working time;
- High radiopacity;
- Optimized mechanical strength;
- Medium viscosity.
- SPINOS can be used for vertebroplasty and kyphoplasty.
- Approved for the augmentation of pedicle screws where bone quality is poor, e.g. in patients with osteoporosis or degenerative or neoplastic changes.
- Short mixing time and fast achievement of application viscosity.
The composition of the polymers ensures a high initial cohesion and therefore reduces the risk of cement leakage. After a short waiting time, the cement attains an ideal viscosity for application. - Long application time.
Both components (powder 24 g and liquid 10 ml) bind quickly to a homogenous paste with a suitable viscosity for percutaneous injection.
After a short mixing time, the surgeon has sufficient time for the transfer of SPINOS into the application instruments, followed by a long application time. - High radiopacity with 45% ZrO2.
The addition of zirconium dioxide (ZrO2) allows an optimal X-ray visualization of SPINOS for safe use. - Good fatigue strength.
The composition of SPINOS guarantees optimized mechanical properties which exceed the respective requirements of the ISO 5833 standard.
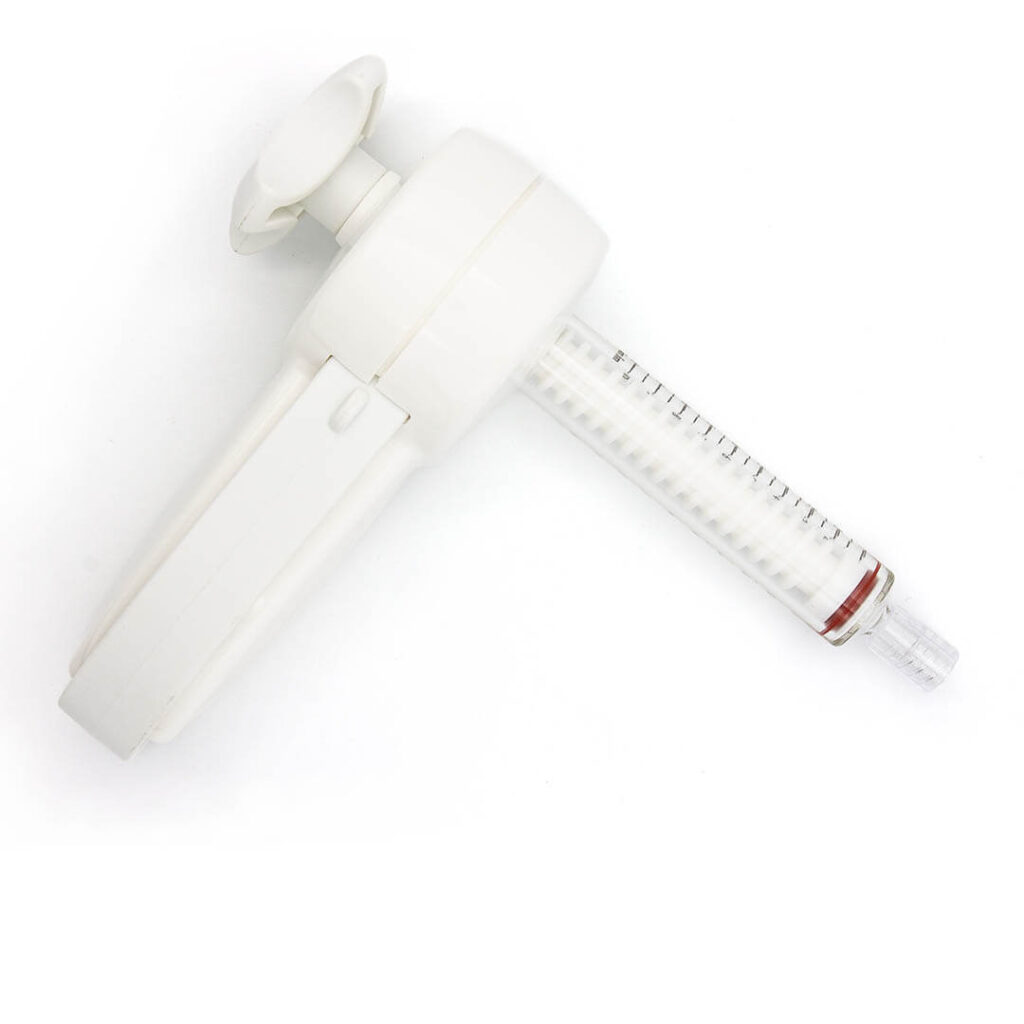
Thanks to its medium viscosity, SPINOS can be used with suitable approved PMMA cement application tools.
SPINOS order code: 01-350 (valid worldwide, except in the U.S.)
RELATED PRODUCTs
Advanced cement delivery system
Bone cement mixing system
Vertebroplasty needle – Steel handle
Vertebroplasty needle – Plastic Handle