UNLUX SYSTEM™
Anti-dislocation bone marrow biopsy needle
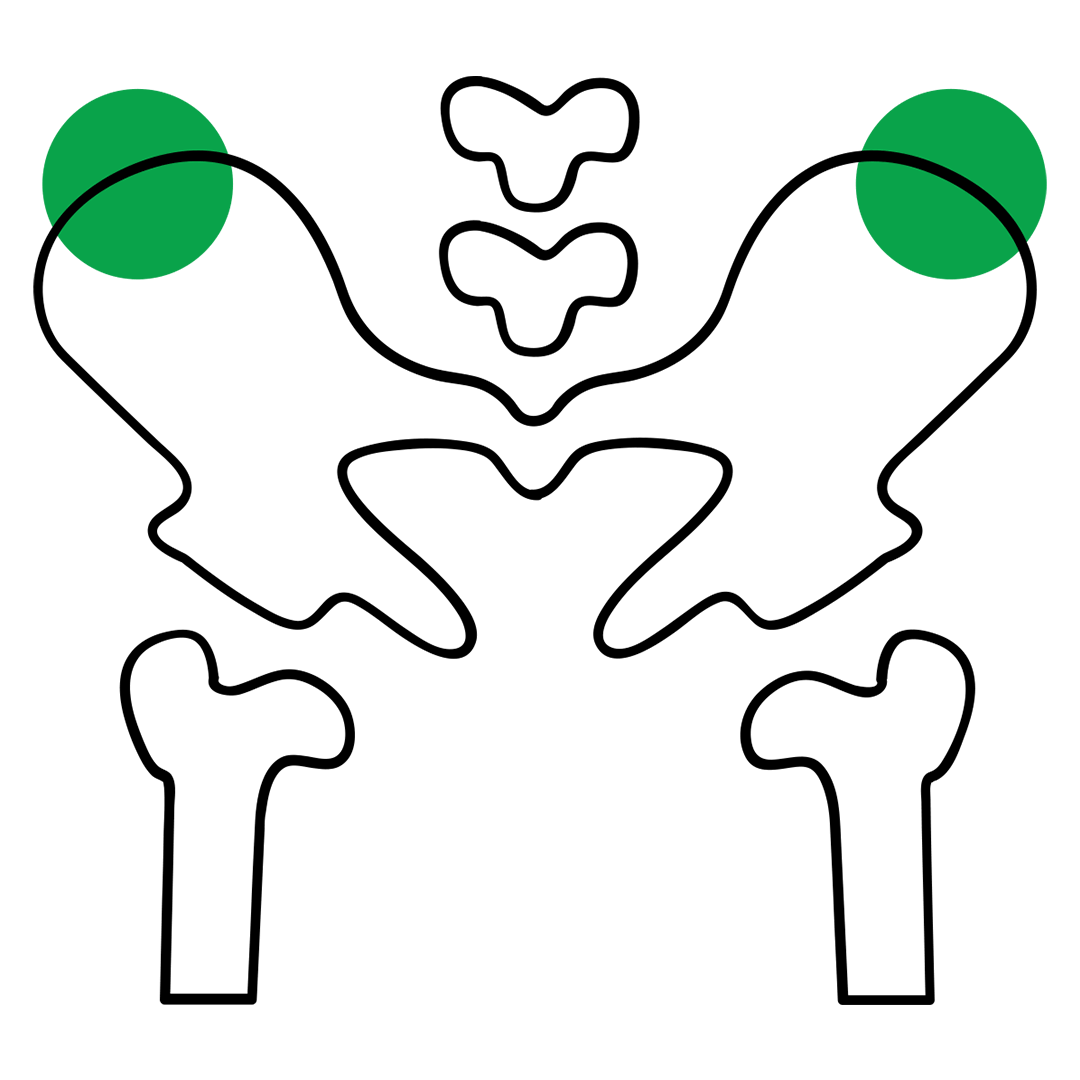
The UNLUX SYSTEM™ needle allows users to perform bone marrow biopsies on the iliac crest, avoiding dislocation manoeuvre.
- The “Trocar” tip stylet guarantees fast and less traumatic penetration.
- The ergonomic handle improves grip and the precision of the biopsy operation.
- With the universal Luer-Lock connector, any syringe can be connected.
- The ultra-sharp crown tip of the cannula facilitates the removal of an intact bone marrow fragment.
The UNLUX SYSTEM™ needle allows users to perform bone marrow biopsies on the iliac crest. With this particular system, it is possible to avoid the dislocation manoeuvre when the sample is being withdrawn. Due to its particular features, specific technical aspects of this device have been patented.
Surgical technique:
- Insert the cannula + stylet system perpendicular to the bone surface. Advance by pushing and twisting the needle left and right to the medullary cavity.
- Remove the stylet.
- Advance 2 cm.
- Insert the UNLUX SYSTEM™ cutter and rotate the cutter + cannula system 360° twice to cut the tissue without damaging it and without dislocation.
- Remove the cannula + cutter system from the patient.
- To extract the specimen, insert the finger guard from the tip side, remove the UNLUX SYSTEM™ cutter and insert the extractor from the tip side.
| GAUGE | DIAMETER (mm) | PRODUCT CODE | NEEDLE SIZE | PIECES PER BOX |
| 8G | 4,00 | ULSEC0810C | 8G x 10cm | 10 |
| ULSEC0815C | 8G x 15cm | |||
| 11G | 3,00 | ULSEC1110C | 11G x 10cm | 10 |
| ULSEC1115C | 11G x 15cm |
RELATED PRODUCTs
Anti-dislocation bone marrow biopsy needle
Anti-dislocation bone marrow biopsy needle
Trocar-tip Jamshidi needle for bone marrow biopsy