PAB™
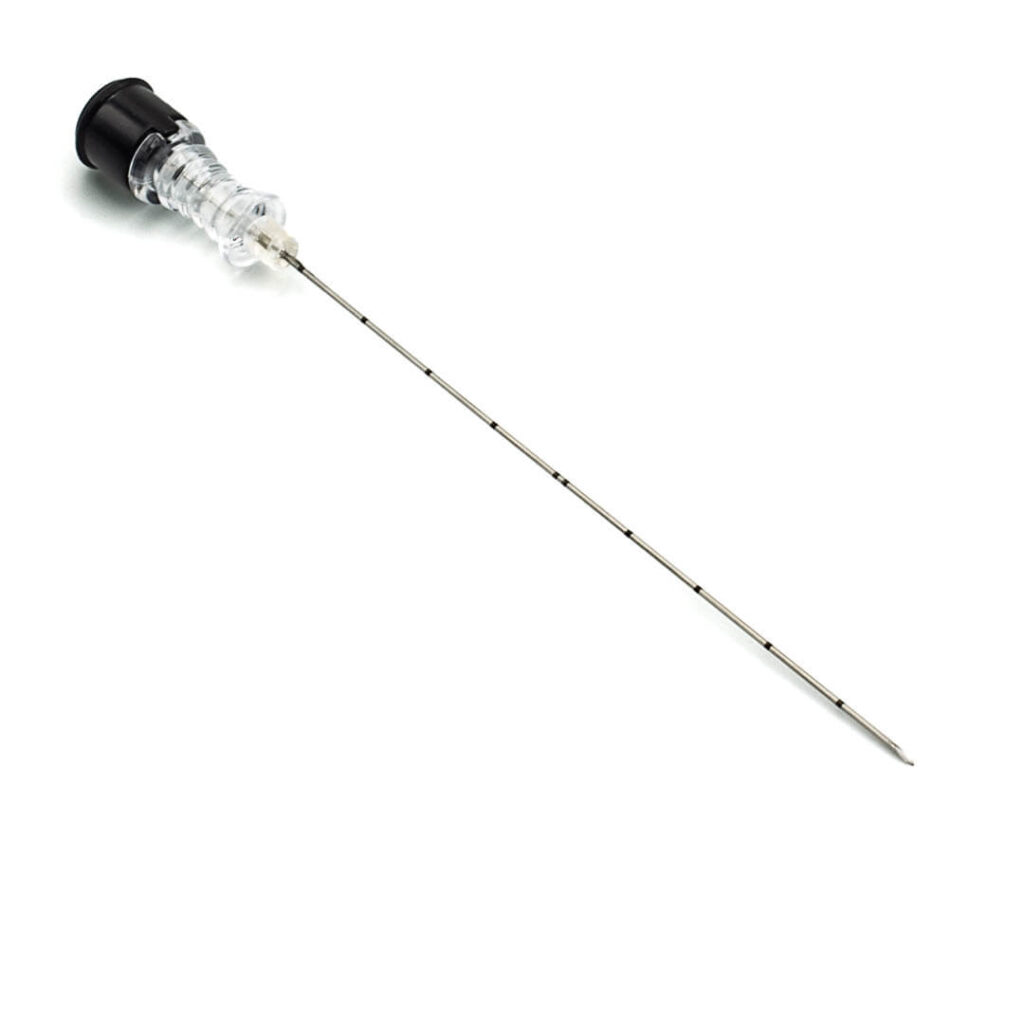
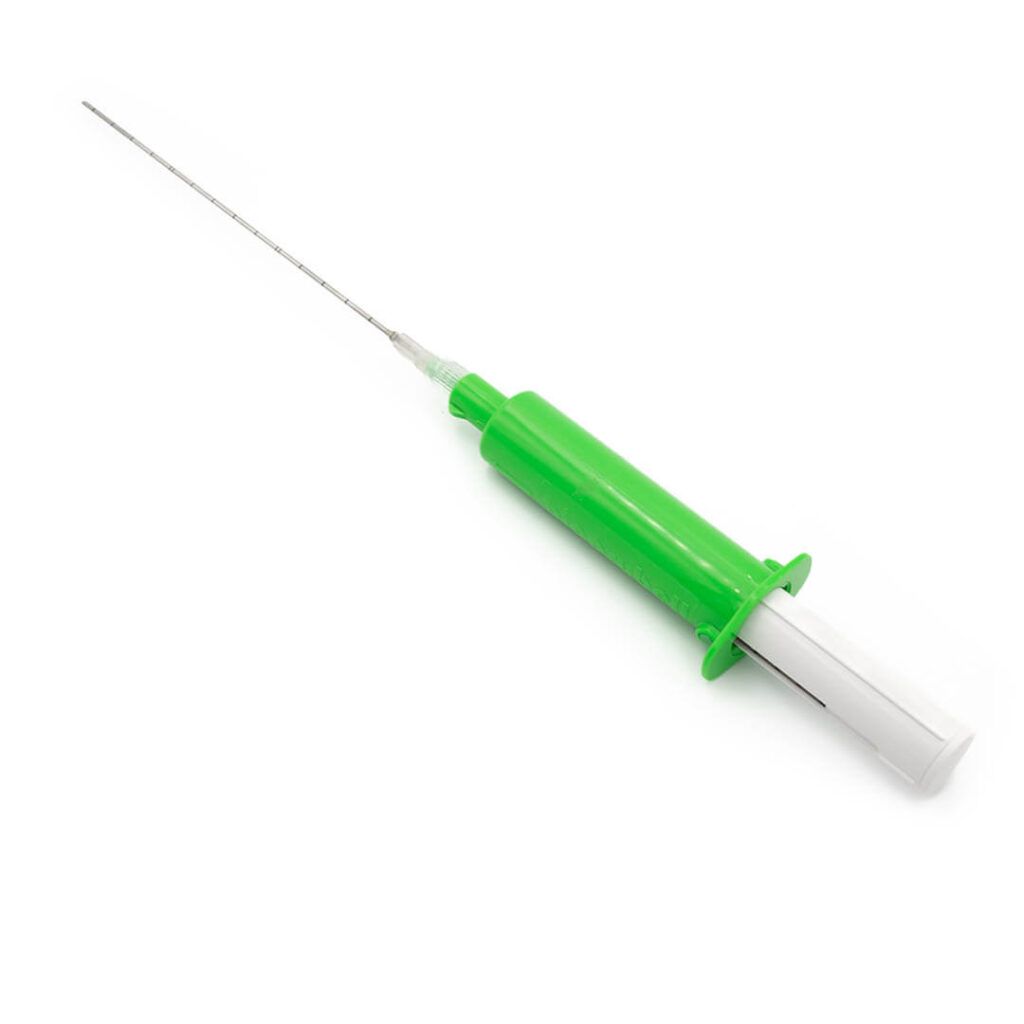
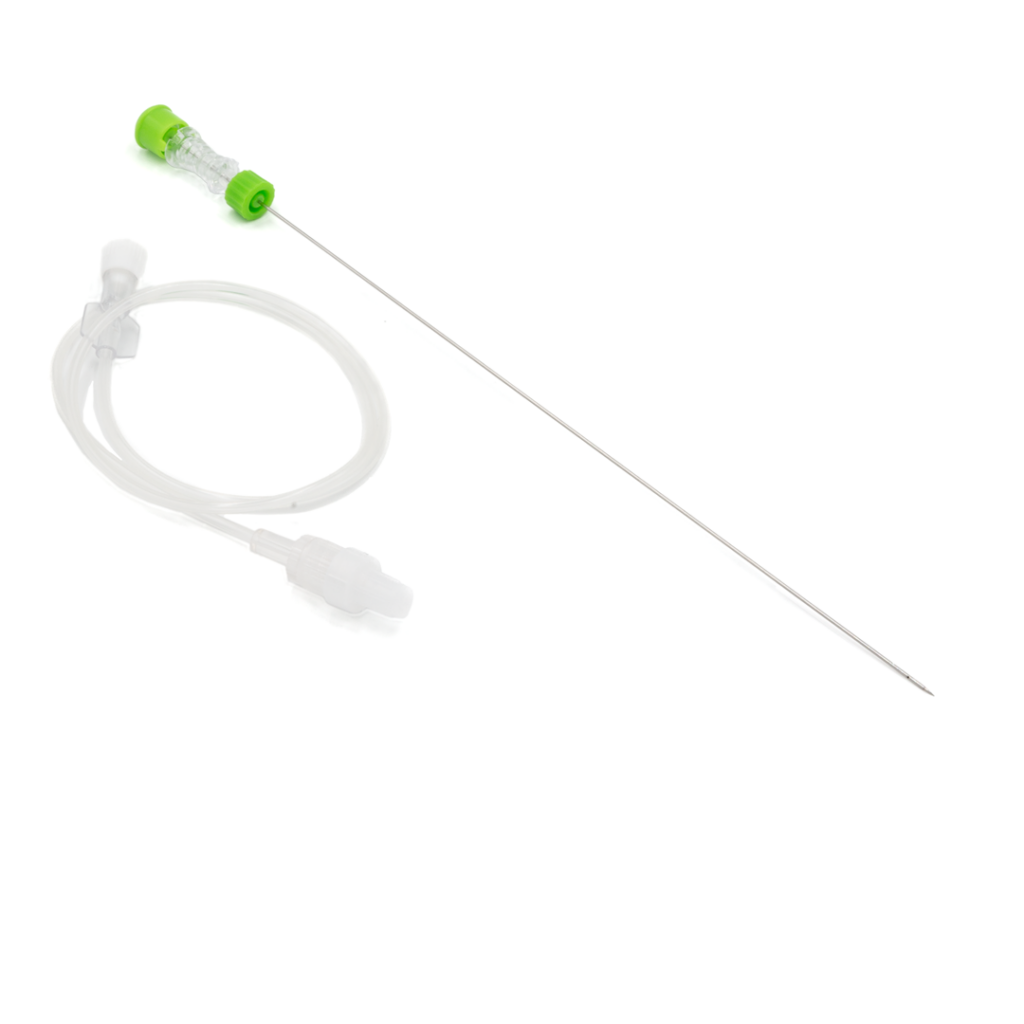
Tumor alcoholization needle
PAB™ is used for the injection of ethyl alcohol directly into the tumour interstice, causing tumour necrosis.
- Offset perforations ensure even alcohol diffusion for complete tumour necrosis.
- The particular sharpness of the trocar tip improves penetration.
- The internal stylet offers rigidity during penetration.
- Equipped with a Luer-lock universal connection and a 50 cm extension.
- The passage of liquids can be seen through the transparent plastic handle.
- Multiple configurations are available: with 3, 5 or 9 holes for tailored diffusion.
Ethanol ablation, also called percutaneous ethanol injection (PEI), is a minimally invasive procedure used to treat certain types of tumours and cysts by injecting absolute ethanol (95-99% alcohol) directly into the lesion.
The ethanol induces cell dehydration, coagulative necrosis, and vascular thrombosis, leading to tissue destruction.
Ethanol ablation remains a cost-effective and minimally invasive option in selected cases, especially when other options are not feasible.
Surgical technique:
- Identify the precise target area for alcoholization.
- Insert the PAB™ needle into the designated site.
- Upon reaching the target, remove the stylet and connect the extension.
- Using a Luer-lock syringe, inject ethanol gradually.
- Once alcoholization is complete, detach the syringe and retract the needle.
| GAUGE | DIAMETER | LENGTH | CODE | PIECES PER BOX |
| 21 | 0,80 MM | 15 | PB211508P50 | 20 – with 3 holes |
| 21 | 0,80 MM | 15 | PB211512P50 | 20 – with 5 holes |
| 21 | 0,80 MM | 20 | PB212008P50 | 20 – with 3 holes |
The 9-hole version is available upon request as a custom-made item.
RELATED PRODUCTs
Automatic device for soft tissue biopsy
Semi-automatic guillotine needle for soft tissue biopsy
Aspiration needle for soft tissue biopsy and prenatal diagnosis
Automatic aspiration device for histological biopsy