SUBCHONDRAL BONE MARROW LESIONS TREATMENT
HOME
>
SURGICAL PROCEDURES
>
ORTHOBIOLOGICS PROCEDURES
>
SUBCHONDRAL BONE MARROW LESIONS TREATMENT
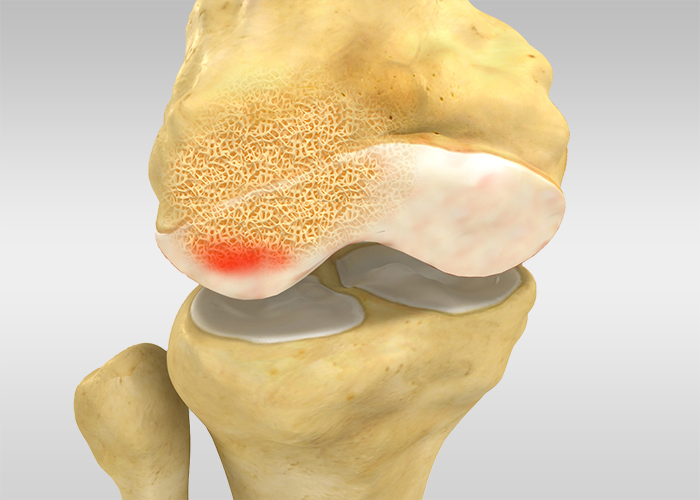
Addressing subchondral bone marrow lesions (BMLs) and avascular necrosis is paramount within the realm of orthopaedics and musculoskeletal health.
Often detected through MRI imaging, these conditions signal an array of underlying issues, from osteoarthritis to traumatic injuries and even certain cancers. Frequently affecting weight-bearing joints, they can inflict pain and hinder mobility.
Enter ORTHOPLASTY™, an innovative surgical toolkit specially designed to combat subchondral bone marrow oedema.
Employing a minimally invasive, percutaneous approach akin to vertebroplasty, this toolkit enables the precise delivery of autologous bone marrow or cutting-edge synthetic biomaterials.
This method significantly reinforces subchondral bone, offering several advantages such as reduced infection risk, rapid functional recovery, pain alleviation within a few days, and the presentation of anatomical integrity across various joints for future interventions. The provided synthetic material is biologic, radio-opaque, and bioresorbable, hardening solely in wet environments. Its ultimate compressive strength surpasses that of healthy cancellous bone, ensuring durability.
SURGICAL PROCEDURE
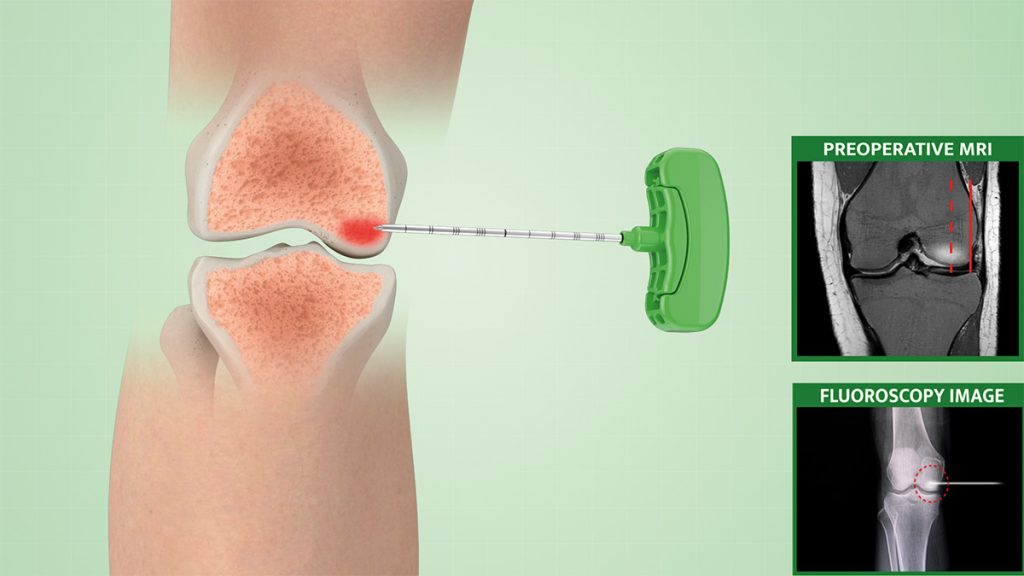
1.
The patient is positioned on the surgical bed under mild sedation.
Under real-time imaging, the surgeon starts by using a working cannula with a trocar tip to identify and access the affected area, and a drill is used to reach the desired depth.
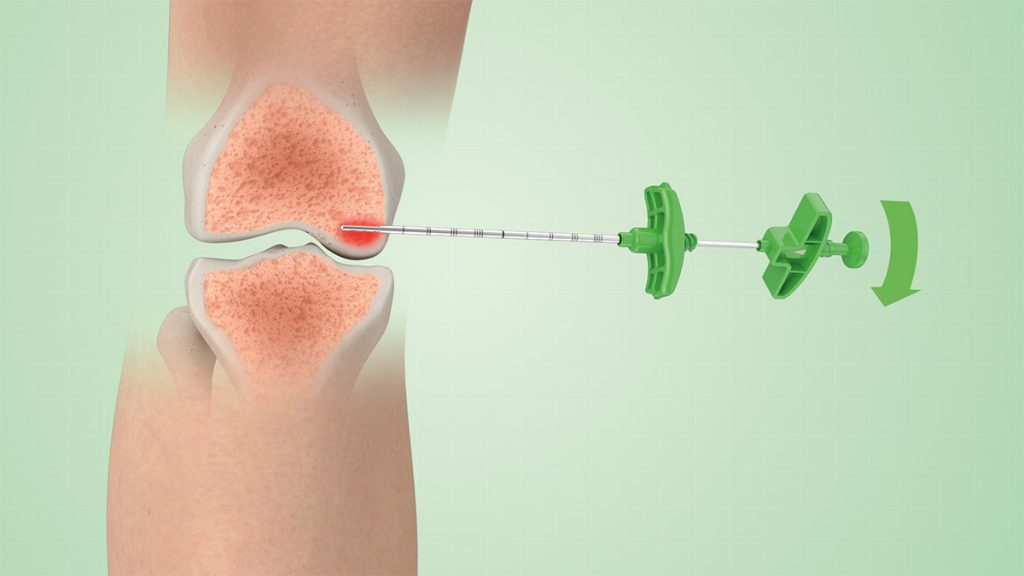
2.
A selective-release bone filler is then inserted into the lesion and filled with the synthetic biomaterial or a pre-chosen bone substitute.
The selective-release bone filler consists of a stylet + steel cannula equipped with lateral holes, to allow a directable injection of the biomaterial in the area to be treated.
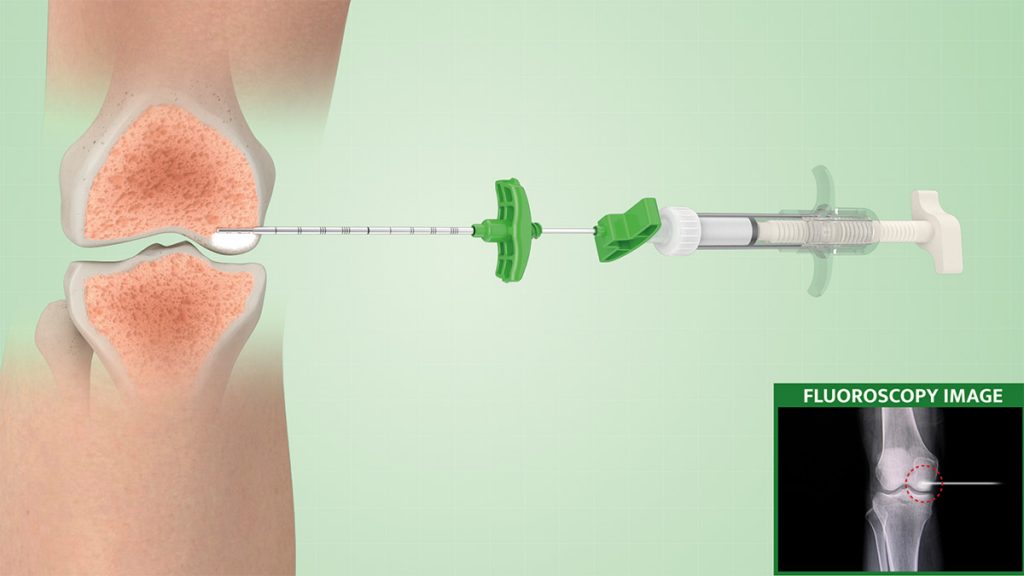
3.
The whole procedure and the quantity of injected materials are monitored with continuous X-ray monitoring to target and ensure the filling of the whole lesion.
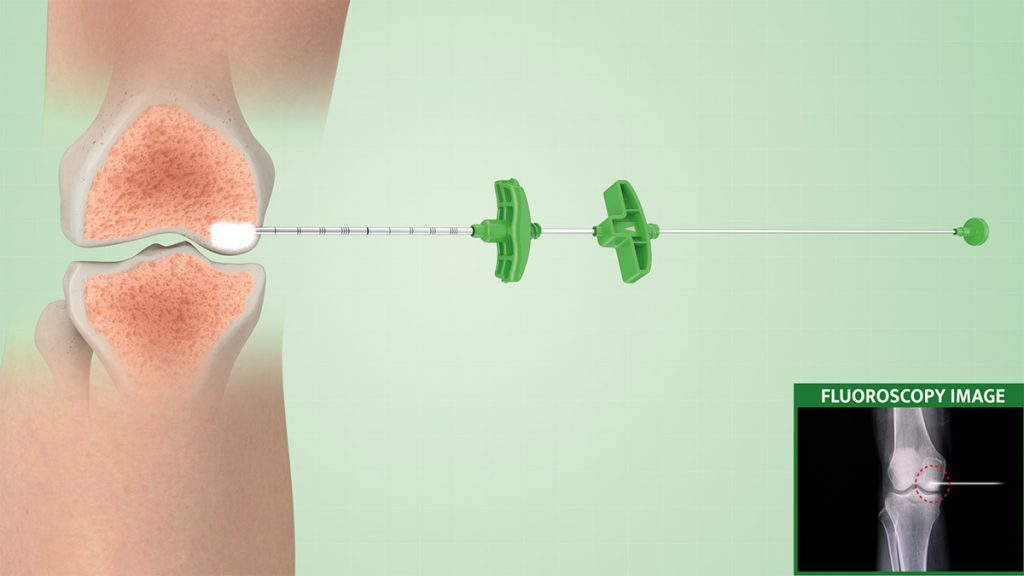
4.
We recommend always checking to empty the selective-release bone filler before removing the access trocar.
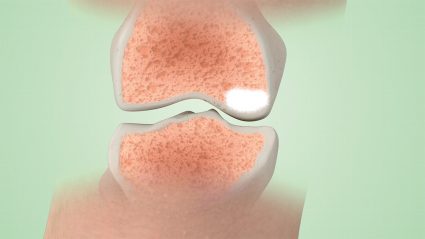
5.
A post-treatment arthroscopy check will allow the removal of any possible leakage.
VIDEO TUTORIAL
Fill out the form for more information.