RENOVA SPINE™ 11G:
kyphoplasty kit for fast approach
RENOVA SPINE™ 8G:
kyphoplasty kit for classic approach
Home » SPINE SURGERY PROCEDURES » KYPHOPLASTY PROCEDURE
A vertebral compression fracture (VCF) occurs when a vertebral body collapses due to osteoporosis, trauma, or tumors such as multiple myeloma.
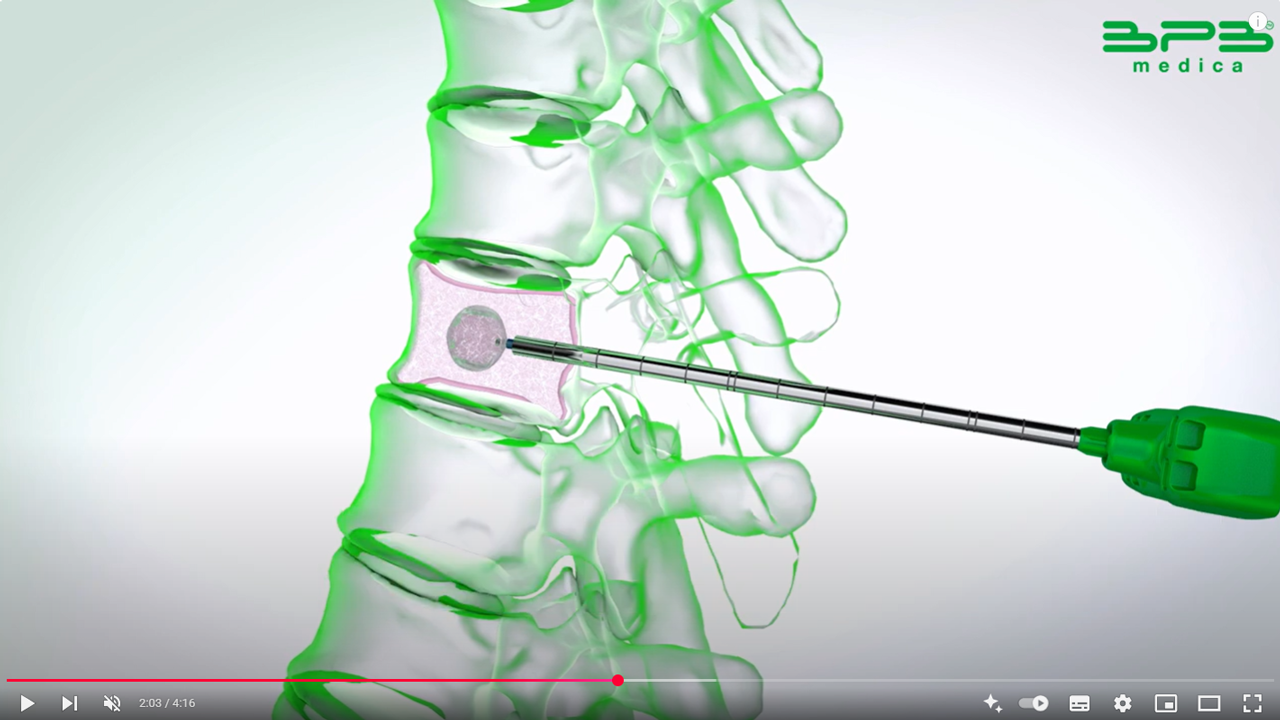
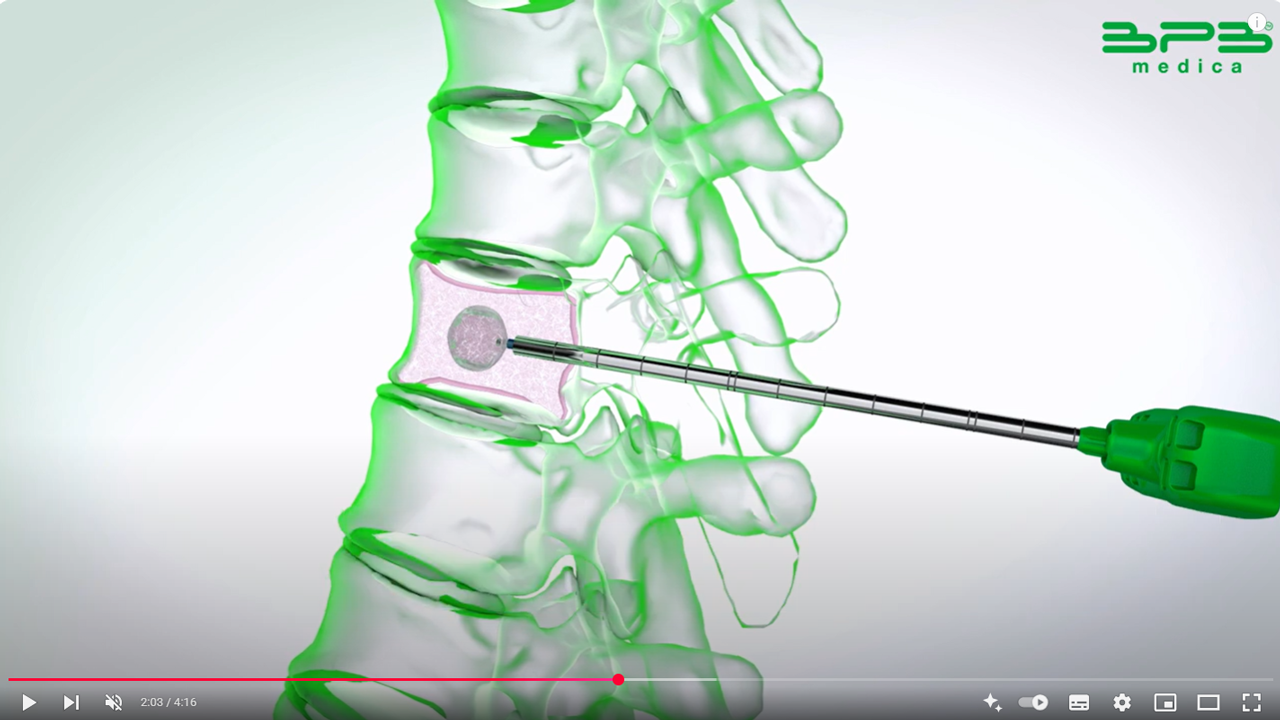
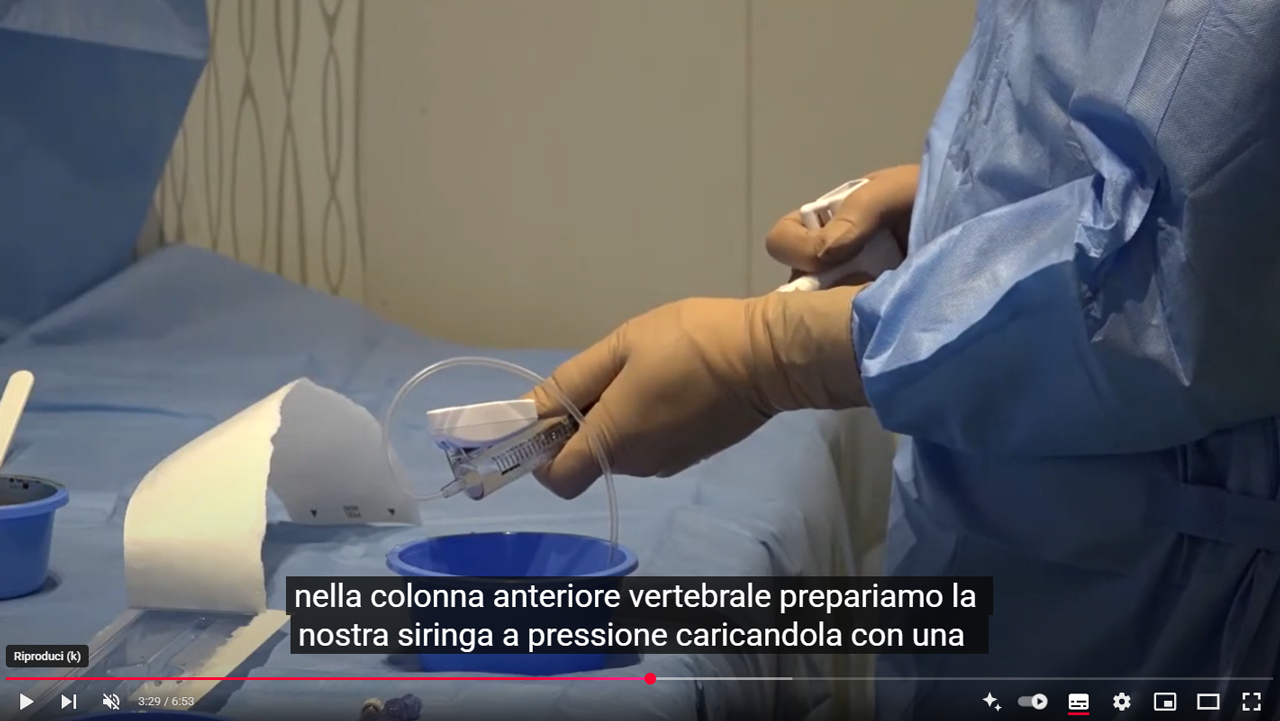
Kyphoplasty aims to gently and progressively restore vertebral height by inflating balloons within the vertebral body. This process creates a cavity by compacting the cancellous bone, which helps minimize the risk of cement leakage. Once the balloons are deflated and removed, the cavity is filled with bone cement to stabilize the fracture.
RENOVA SPINE™ is a minimally invasive system designed for kyphoplasty procedures. It helps treat vertebral fractures, restore vertebral height, and relieve back pain. The system facilitates cavity creation in the spongy bone and is intended for use with a legally marketed bone void filler suitable for vertebroplasty or vertebral augmentation.
Two access options are available:
Working cannula with trocar or bevel tip: enables direct percutaneous access with fewer surgical steps; included in the RENOVA SPINE™ 11G and 13G Kyphoplasty kits.
Guidewire access: guides the introduction of instruments; included in the RENOVA SPINE™ 8G Kyphoplasty kit.
Indications
• Painful vertebral compression fractures
• Osteolytic lesions within the vertebral body
It is possible to perform a transpedicular or extrapedicular access, depending on the anatomy of the vertebral body to be treated.






Fill out the form below, one of our expert will get in touch with you shortly!
The content of this website is intended exclusively for healthcare professionals.
In accordance with current regulations (Legislative Decree 46/97 and EU Regulation 2017/745), access to information related to medical devices is restricted to healthcare professionals only.
I declare that I am a healthcare professional.