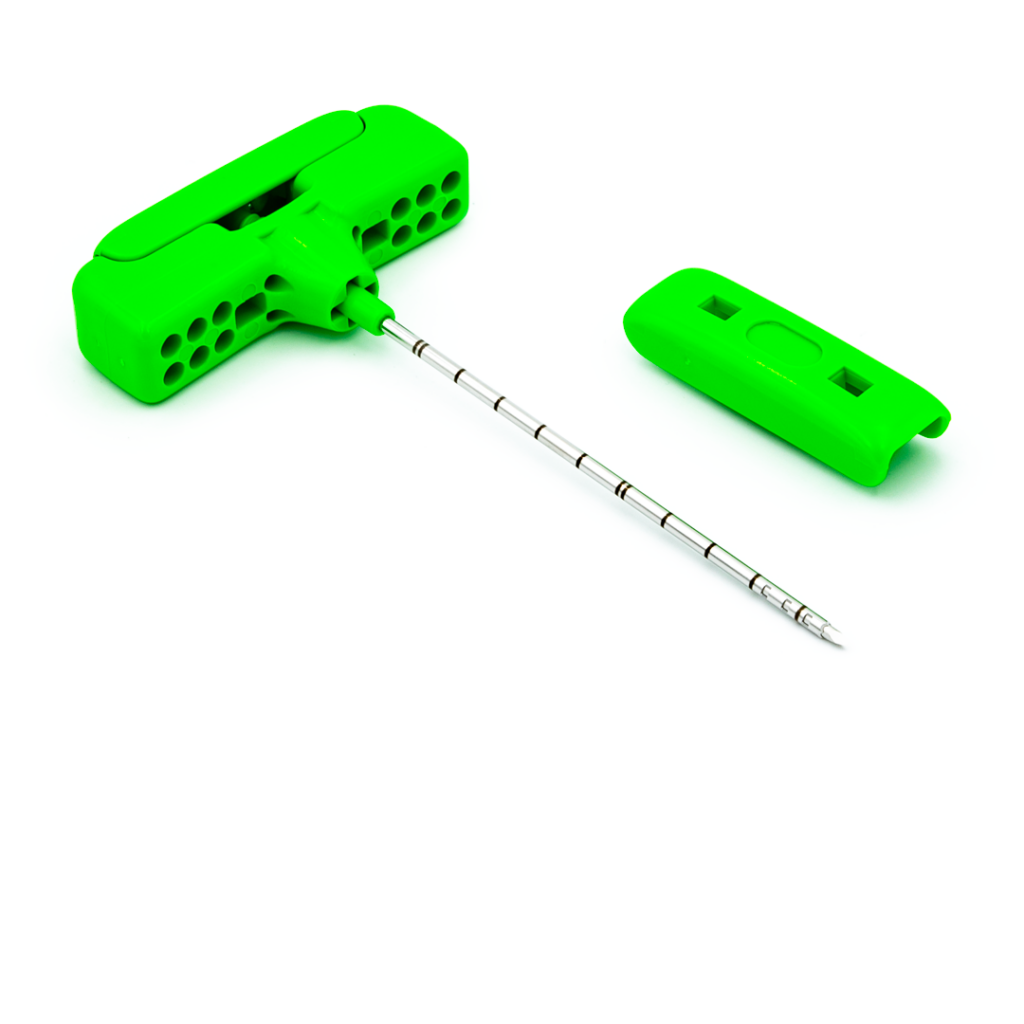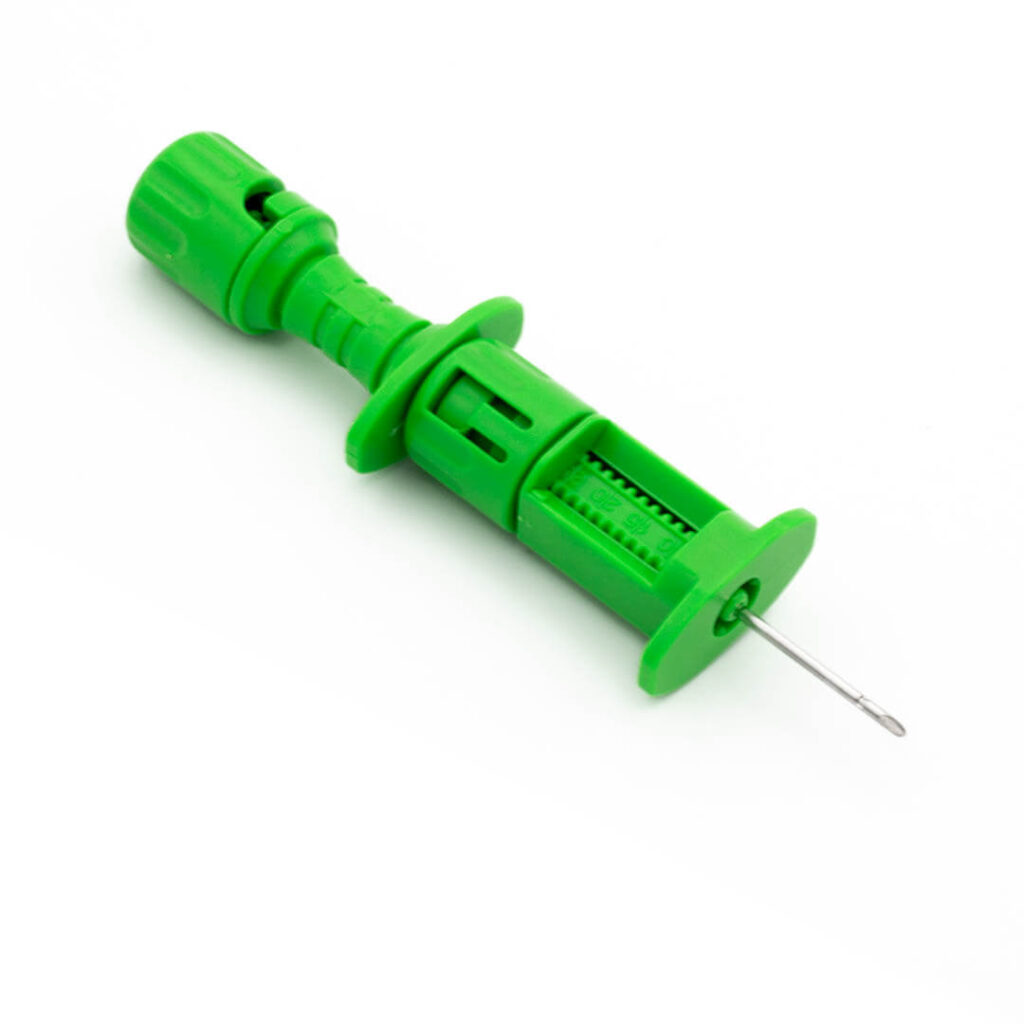
STERNOBELL EXPLANT™
Bone marrow harvest needle
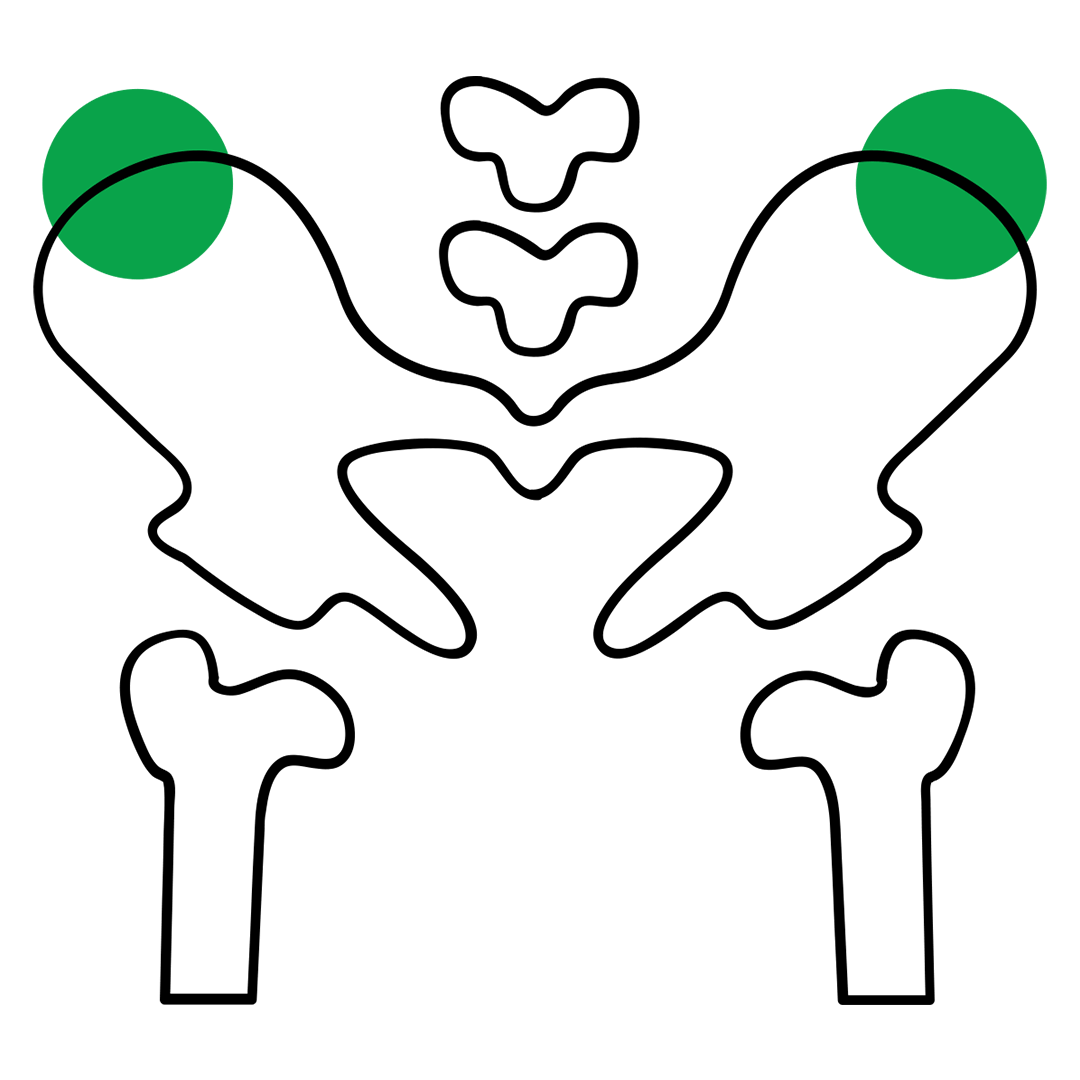
STERNOBELL EXPLANT™ is a bone marrow harvest needle designed for allogeneic and autologous bone marrow harvest transplantation procedures.
- The two holes on the cannula are offset from each other for a quicker and greater aspiration of bone marrow and hematopoietic cells.
- Ergonomic handle.
- The millimetric advancement ring facilitates the adjustment of the length of the needle, which indicates the achievable depth.
- The triple sharpened cannula enables an easy and less traumatic penetration.
- With the universal Luer-Lock connector, any syringe can be connected.
- It is possible to completely unscrew the millimetric advancement system to make use of the total length of the needle.
Surgical technique:
- Adjust the protrusion of the cannula by turning the ring nut clockwise. The measurement is visible on the millimetric scale.
- If necessary, remove the ring + depth stop system to use the entire available length.
- Position the cannula + stylet system on the selected site, pass through skin and subcutis, and push by turning left and right until penetrating the bone cavity at the selected depth.
- Unscrew the cap and remove the stylet. Attach a syringe and proceed to aspirate the bone marrow blood.
- Disconnect the syringe with the aspirated blood.
- Reinsert the stylet into the cannula and remove the needle from the patient’s body by turning it to the right and left.
| GAUGE | DIAMETER (mm) | PRODUCT CODE | NEEDLE SIZE | PIECES PER BOX |
| 14G | 2,10 | RNE1455 | 14G x 5,5cm | 20 |
| 15G | 1,80 | RNE1540 | 15G x 4,0cm | 20 |
| RNE1555 | 15G x 5,5cm |
RELATED PRODUCTs
Bone marrow harvest needle with T handle