STERNOBELL™
Needle for bone marrow aspiration
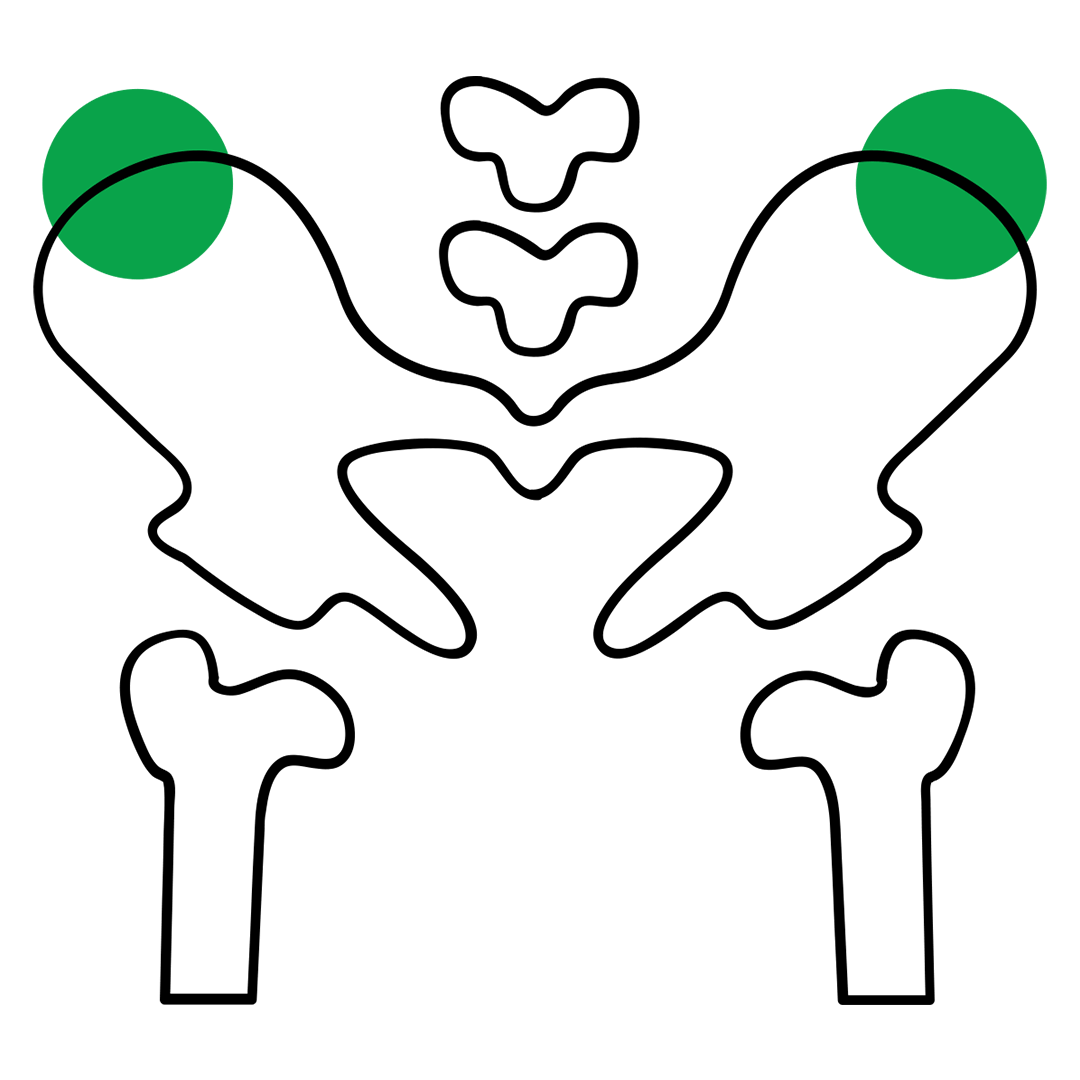
The STERNOBELL™ needle is a single-use device for bone marrow aspiration with millimetric advancement system, ideal for sternal or iliac crest access.
- Ergonomic handle.
- The millimetric advancement ring facilitates the adjustment of the length of the needle, which indicates the achievable depth.
- The triple sharpened cannula enables an easy and less traumatic penetration.
- With the universal Luer-Lock connector, any syringe can be connected.
- It is possible to completely unscrew the millimetric advancement system to make use of the total length of the needle.
Surgical technique:
- Adjust the cannula by turning the millimetre advancement ring. The size of the protrusion can be seen on the graduated scale.
- Insert the cannula + stylet system passing through skin and subcutis to the predetermined depth of the bone cavity.
- Unscrew the cap, remove the stylet and connect a Luer-lock syringe. Proceed with aspiration of the bone marrow blood.
- Disconnect the syringe with the aspirate and reinsert the stylet.
- Remove the cannula + stylet system with rotary movements.
| GAUGE | DIAMETER (mm) | PRODUCT CODE | NEEDLE SIZE | MINIMAL LENGTH (mm) | MAXIMAL LENGTH (mm) | MAXIMUM LENGTH WITHOUT GEAR (mm) | PIECES PER BOX |
| 14G | 2,10 | RN1425 | 14G x 2,5cm | 7 | 25 | 32 | 20 |
| RN1440 | 14G x 4,0cm | 22 | 40 | 47 | |||
| RN1455 | 14G x 5,5cm | 37 | 55 | 62 | |||
| 15G | 1,80 | RN1525 | 15G x 2,5cm | 7 | 25 | 32 | 20 |
| RN1540 | 15G x 4,0cm | 22 | 40 | 47 | |||
| RN1555 | 15G x 5,5cm | 37 | 55 | 62 | |||
| 16G | 1,60 | RN1625 | 16G x 2,5cm | 7 | 25 | 32 | 20 |
| RN1640 | 16G x 4,0cm | 22 | 40 | 47 | |||
| RN1655 | 16G x 5,5cm | 37 | 55 | 62 | |||
| 18G | 1,20 | RN1825 | 18G x 2,5cm | 7 | 25 | 32 | 20 |
| RN1840 | 18G x 4,0cm | 22 | 40 | 47 | |||
| RN1855 | 18G x 5,5cm | 37 | 55 | 62 |
RELATED PRODUCTs
Bone marrow aspiration needle with T handle