Home » Products » Assisted reproduction technology » TRANSFER OMNI-S/L
TRANSFER OMNI-S/L
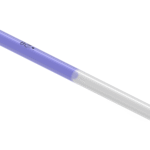
Embryo transfer catheter for “difficult” transfers.
Embryo transfer catheter for “difficult” transfers. Recommended for cases of cervical stenosis thanks to its flexible stainless-steel mandrel. The embryo transfer set is composed of a blue-coloured outer introducer, a malleable mandrel allowing catheter shaping once it is inserted and an embryo transfer catheter.
- Soft and clear distal tip, tapered for ease of insertion
- Blue-coloured outer introducer with graduated markings placed at 5-6-7-8 cm from the tip
- Malleable mandrel allowing catheter shaping once it is inserted.
- Rigid proximal portion of the mandrel
- Luer-lock connector that serves as finger grip
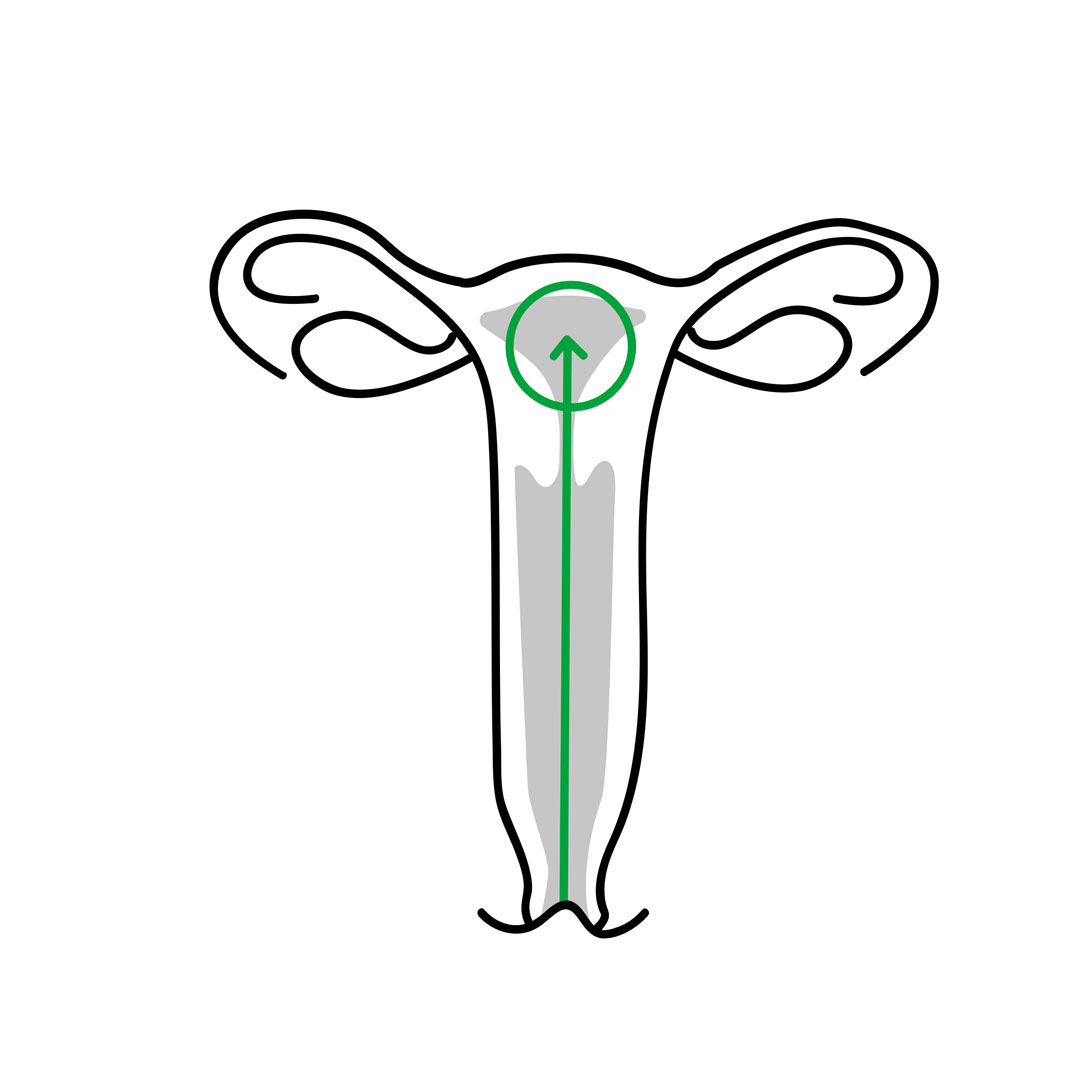
Surgical technique:
- Introduce the speculum, expose the cervix, and if possible, gently remove the cervical mucus. Lightly swab the uterine cervix with a gauze soaked in embryo culture medium.
- Introduce the guide-catheter (with mandrel inside) through the cervix just beyond the internal cervical opening.
- Remove the mandrel from the guide-catheter. Gently advance the transfer catheter (previously connected to the insulin syringe and loaded with embryos) through the guide-catheter inside the uterine cavity until reaching the pre-selected point.
- Do not touch the fundus to avoid any stimulation of the uterus’s contractility.
- Once the embryo transfer has been completed, remove both catheters simultaneously and check under a stereomicroscope for any retained embryo.
| INTRODUCER CATHETER OUTER DIAMETER (mm) | TRANSFER CATHETER OUTER DIAMETER (mm) | PRODUCT CODE | NEEDLE LENGTH INTRODUCER + TRANSFER (mm) | PIECES PER BOX | |
| 2,00 | 1,00 | 770407 | 140 + 210 | TRANSFER OMNI-S | 12 |
| 2,00 | 1,00 | 770406 | 200 + 250 | TRANSFER OMNI-L | 24 |