Home » Products » Assisted reproduction technology » KINDER DL™
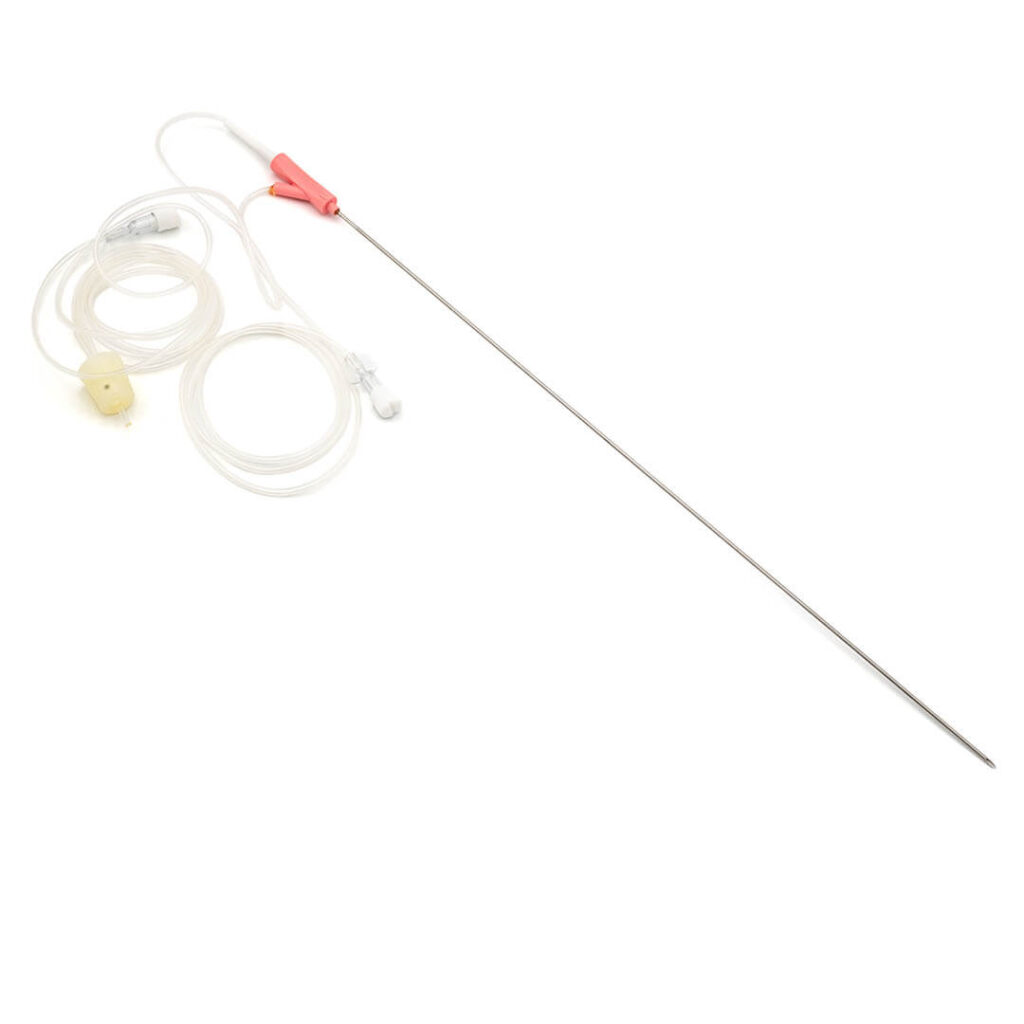
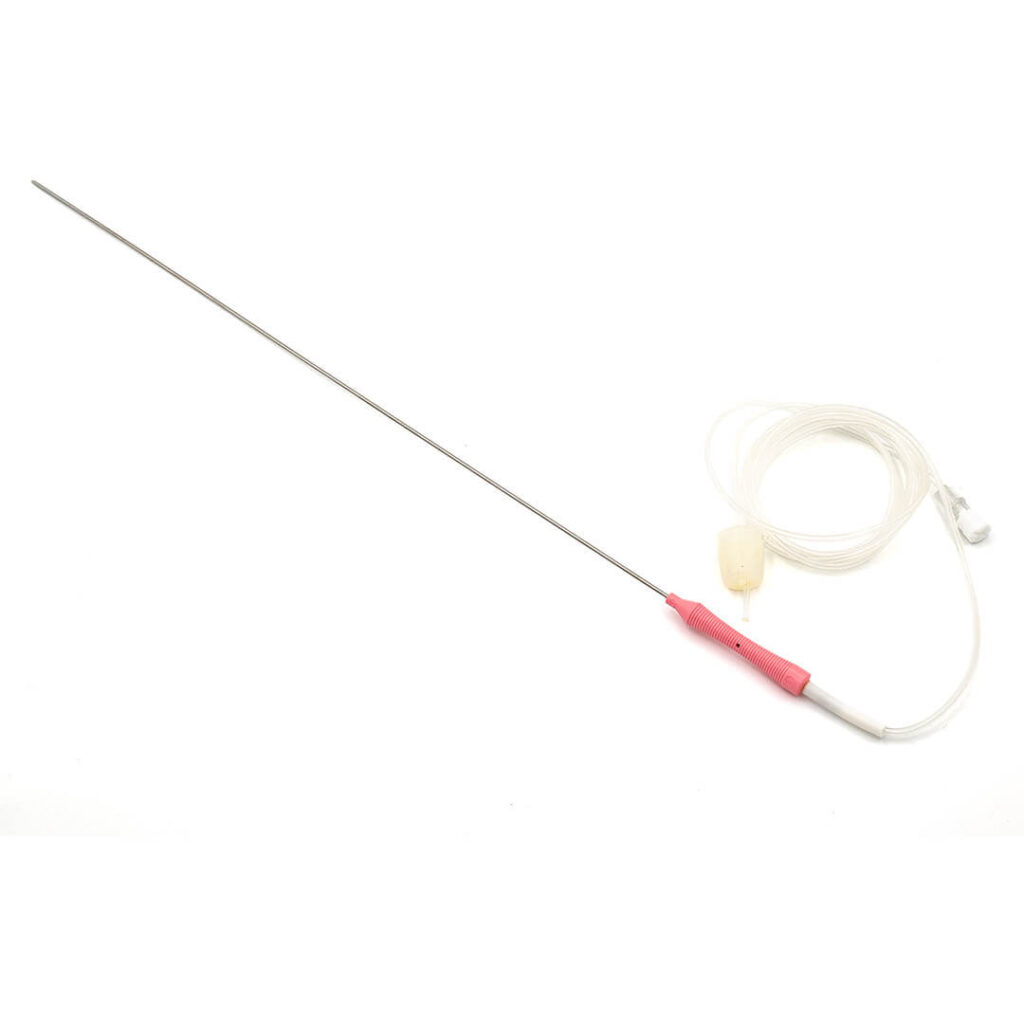
KINDER DL™
Double lumen oocyte aspiration needle
- Direct connection between needle and aspiration line;
- Ergonomic grip with thumb notch to indicate the orientation of the tip;
- Echo markings for accurate placement under ultrasound guidance;
- Triple cut tip to reduce trauma;
- Different measures available upon request;
- MEA/LAL tested for safety and biocompatibility.
Oocyte retrieval is a crucial step in Assisted Reproduction Technology (ART), involving the collection of eggs from ovarian follicles. A double-lumen aspiration needle enhances this procedure by allowing both the aspiration of follicular fluid and its controlled flushing.
Under ultrasound guidance, the needle is inserted into the ovarian follicles. The first lumen is used for aspiration, drawing out the follicular fluid containing the oocyte, while the second lumen enables the injection of a flushing medium. This method helps to collect all the follicular fluid, ensuring effective collection even in less than ideal conditions. Depending on the clinician’s preference, the flushing and aspiration can be performed simultaneously or intermittently.
This design ensures greater efficiency and precision, optimising the number of viable oocytes collected while minimising trauma to the ovarian tissue.
| GAUGE | DIAMETER (mm) | PRODUCT CODE | Needle Length (cm) | PIECES PER BOX |
| 16G | 1,60 | KKN2V1635E8090 | 35 | 10 |
| 17G | 1,47 | KKN2V1735E8090 | 35 | 10 |