If you have chosen to decline all cookies, you may encounter blank spaces instead of video content.
To enjoy the full video experience, consider adjusting your cookie preferences clicking on the below button.
Thank you for your understanding!
Manage you cookie preferences Close
ORTHOPLASTY™
-
HOME
>
PRODUCTS LINES
>
ORTHO-BIOLOGICS
>
ORTHOPLASTY
ORTHOPLASTY™ is a disposable device for treating subchondral bone lesions.
The subchondral bone plasty procedure is a minimally-invasive, fluoroscopically-assisted procedure that identifies and repairs subchondral bone defects, also named Bone Marrow Lesions (BMLs). The procedure is carried out with a minimally-invasive approach under fluoroscopy guidance along with arthroscopy, to target and manage findings inside the joint.
The pathology is classified as a SIFK (Subchondral Insufficiency Fracture of the Knee) and in the initial stages of SONK (Spontaneous Osteonecrosis of the Knee). The patient that presents with this pathology, suffers from relatively early osteoarthritis and consults the clinical specialist as a result of intense pain that does not correspond to a significantly compromised radiographic scenario.
In fact, these lesions are not visible under X-Ray and only a diagnostic confirmation using MRI reveals a hyper-intense uptake signal in sequences sensitive to T2 fluids (hydrogen) and in STIR sequences.
The objective of the method is to reinforce subchondral bone lesions using the same principle as vertebroplasty and involves the percutaneous insertion into the bone rarefaction site, of an appropriate bone substitute or of an autologous bone graft enhanced with a concentrate of mesenchymal stromal cells.
BENEFITS
Safe and precise minimally-invasive percutaneous approach
Fast procedure: approximately 20 minutes procedure
Rapid functional recovery
Pain relief after 1 day
Preservation of anatomical physiology for future operations
Reduced risk of infections
Ready-to-use bone substitute:
no preparation needed
Hardening in the wet environment only: no time pressure during application
Truly biologic: composed of a microcrystalline, calcium-deficient hydroxyapatite – major bone constituent
High load-sharing properties (up to 45 MPa)
Radio-opaque paste: clearly visible under fluoroscopy and X-rays
Bioresorbable during bone remodelling
APPLICATION
DISTAL FEMUR
PROXIMAL HUMERUS
PROXIMAL TIBIA
ANKLE JOINT
FEMORAL HEAD
HIP
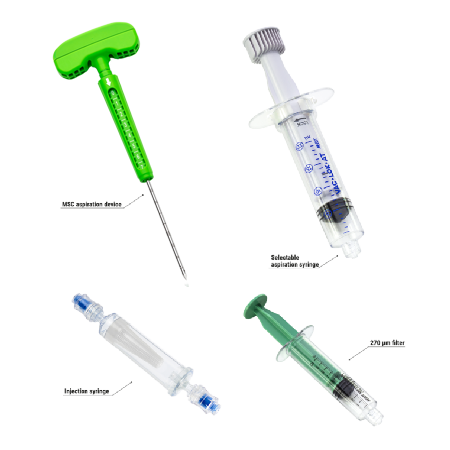
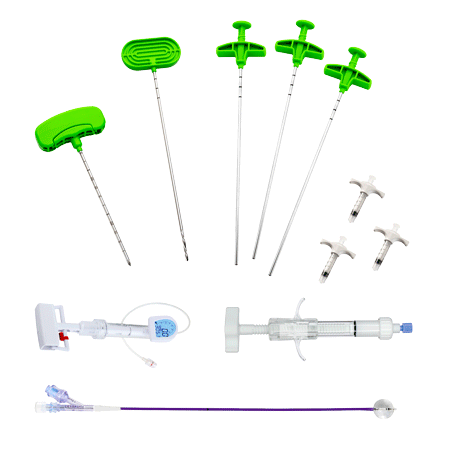
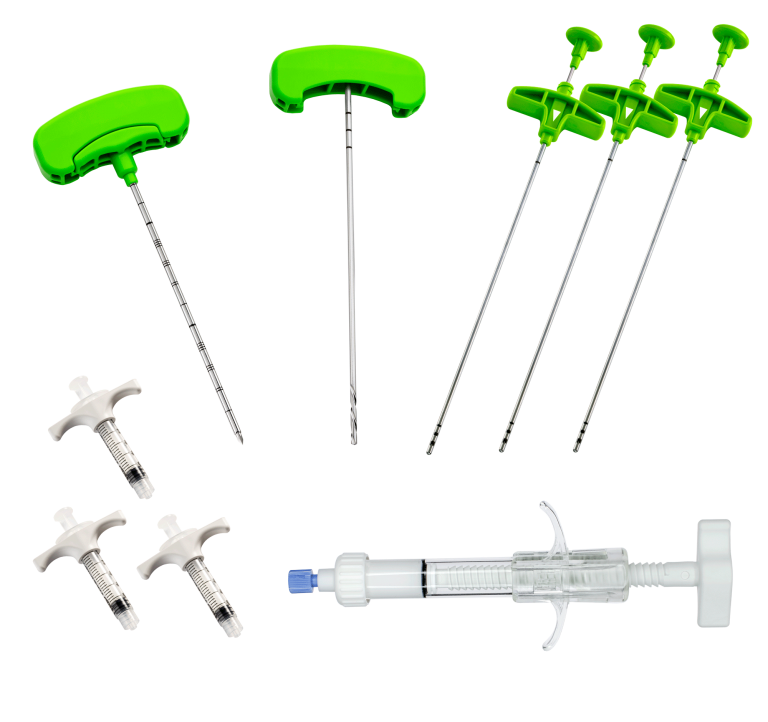
COMPONENTS
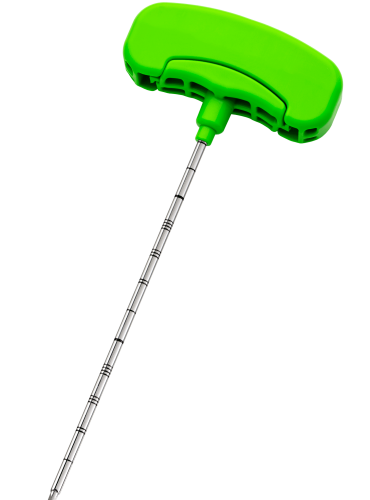
– DIRECTABLE BONE FILLER
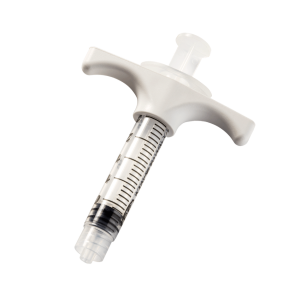
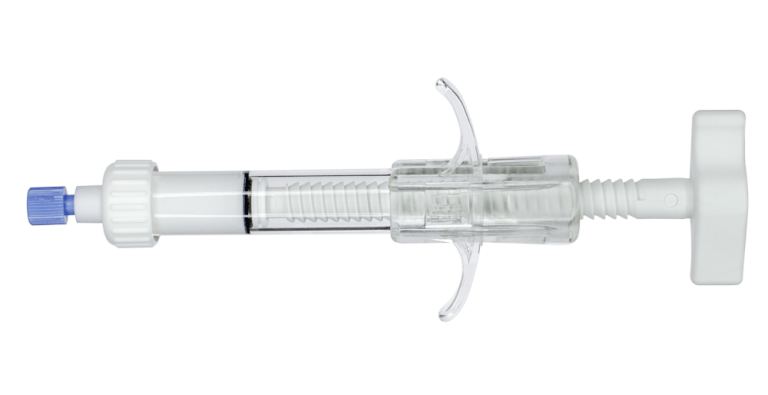
is a cement infusion cannula (often referred to as the “filler” cannula) and consists of a steel cannula with a plastic handle, equipped with a pusher stylet. The lateral holes in the cannula allow a directable injection of the bone cement in the area to be treated. The plastic handle has a universal Luer-lock connection for filling the cannula with bone cement. Also, dedicated injection syringes with Luer-lock connections are present in the kit.
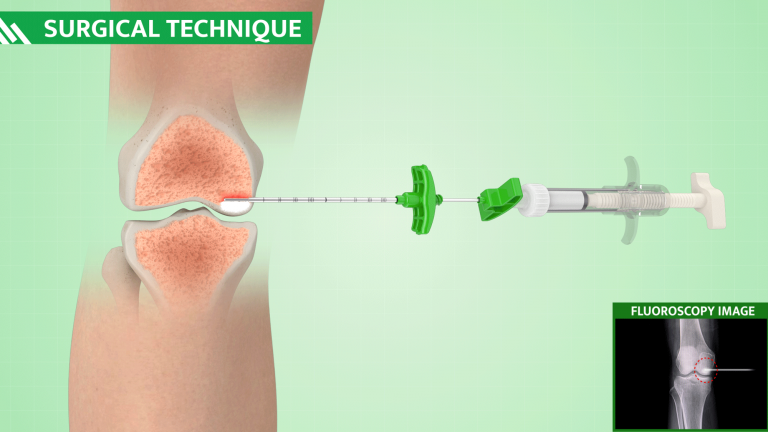
SURGICAL TECHNIQUE
Identify the Bone Marrow Lesion (BML) using a fat-suppressed MRI (T2) and choose the optimal approach and trajectory.
Through intraoperative fluoroscopy, target the defect associated with Bone Marrow Lesion (BML) linked to the MRI results.
Access the bone defect using the ORTHOPLASTY™ access tools kit.
Fill bone defect with bone substitute under fluoroscopic guidance.
Alternatively, BML defects can be filled with a MARROW-STEM™ kit, using the creeping substitution technique and marrow mesenchymal stem cells instead of a bone substitute.